
Know more about ukca marking for medical devices
UKCA stands for United Kingdom Conformity Assessed and is applied to products placed on the market in the UK. This replaces the CE mark, which ? pending changes to legislation - is recognized in the UK until the 30th of June 2023. So from this date onwards, medical device manufacturers need to comply with the new UKCA marking regulations. manufacturers now need to plan for how they will obtain a UK Conformity Assessment mark before 30th June 2023. As many have seen, there have been delays in obtaining the mark, so the quicker manufacturers act, the better. Operon strategist is medical devices consulting has a good experience in consultation and regulatory expertise for manufacturers and suppliers of medical devices. for any medical device registration process, UKCA marking, and licensing you can connect with us. WHAT ARE THE REQUIREMENTS FOR CE-MARKED PRODUCTS IN THE UK? From 1 January 2021, there are a number of requirements that products marketed as medical devices in Great Britain (England, Wales, and Scotland) must comply with: Certificates issued by notified bodies recognized by the EU will remain valid for the UK market until 30 June 2023. The EU no longer recognizes the UK?s notified bodies. UK Notified Bodies cannot issue CE certificates (except for the purposes of the ?CE UKNI? mark, which is valid in Northern Ireland), and have become UK Approved Bodies. A new route to market and product marking is available for manufacturers wishing to obtain UKCA marking for medical devices. From 1 January 2021, all medical devices, including in vitro diagnostic (IVD) medical devices, marketed in Great Britain must be registered with the MHRA. There is a grace period for registration: Class III and Class IIb implantables, and all active implantable medical devices and IVD List A products must be registered by 1 May 2021. Other Class IIb devices and all Class IIa devices and Schedule B IVD products and self-testing IVDs must be registered from 1 September 2021. Class I devices, custom-made devices and general IVDs must be registered from 1 January 2022 Class I devices, custom-made devices and general IVDs that, prior to 1 January 2021, were required to register their devices with the MHRA (i.e. UK-based manufacturers or third country manufacturers with authorised representatives based in Northern Ireland) must continue to register their devices from 1 January 2021. So what is the difference between what you have to do to place a CE Mark on a product versus a UKCA Mark? There are two levels to this answer: The mechanics of UKCA-marking The regulatory basis for conformity assessment The mechanics are that a UK Approved Body will need to perform the conformity assessments that formerly the EU Notified Body (NB) would do. It appears that the two UK-based NBs that are able to do the necessary for medical imaging products will become UK Approved Bodies automatically, so not much change there, except that they will nee
-
Category: Health & Beauty
Important!
There are a lot of advertisers on Advertigo. We cannot check them one by one.
You work hard for your money and you want a company you can rely on when you are buying or selling things. That’s why we want to help you protect yourself from fraud. In this section, you’ll find informative tips and other useful material to stay informed and help reduce your chances of falling victim to scammers.
Please understand that Advertigo.net is a free service to help buyers and sellers (and etc.) find one another. Advertigo.net is not involved in any transactions and can not police the actions of our many users.
Useful links
Similar ads

Best root canal treatment in zirakpur
Experience exceptional root canal treatment in Zirakpur, designed to alleviate dental pain and preserve your
Guptasmileclinic
Breast augmentation surgery in tirupati
Are you Looking for reliable Breast Augmentation Surgery in Tirupati? IKON Hospital is your trusted
Amadhu

Online self confidence counselling
Boost your self-esteem with Online Self-Confidence Counseling! Overcome self-doubt, build a positive mindset, and unlock
My Fit Brain
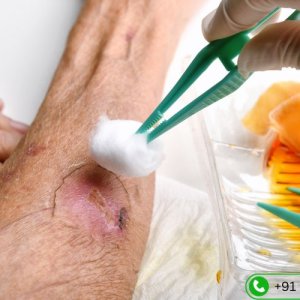
Diabetic wound care specialist in hyderabad | vascularhyd
Dr. Rahul Agarwal is a trusted Diabetic Wound Care Specialist in Hyderabad, providing expert care
Vascularhyd
Best plastic surgeon in manikonda, hyderabad | rex super special
Rex Super Speciality Hospital is the best plastic surgery hospital in Hyderabad, offering advanced treatments
Amadhu
Ayurvedic clinic near me in new friends colony
Jeena Sikho Lifecare Ltd. in New Friends Colony provides excellent Ayurvedic OPD (Out-Patient Department) services...
New Friends Colony

Best height growth capsules for natural growth
Ever wished for a little extra height? You’re not alone! Height Growth Capsules are packed
Kayashreeheightdetox

Pnk beauty: best moisturizer for glowing skin in every season
Achieve radiant skin all year round with Pnk Beauty s Best Moisturizer for Glowing Skin...
Pnk Beauty
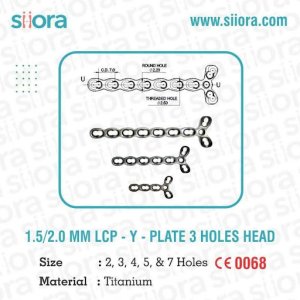
15/20 mm lcp adaption plate
1.5/ 2.0 mm LCP Adaption Plate is intended for the temporary fixation and stabilization of
Siora Surgicals Pvt. Ltd.
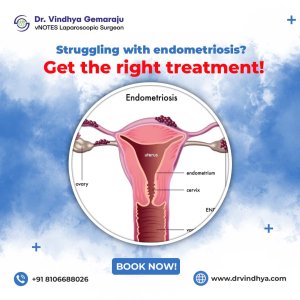
Best endometriosis treatment in hyderabad | dr vindhya gemaraju
If you re looking for the best endometriosis treatment in Hyderabad, Dr. Vindhya Gemaraju provides
Dr Vindhya Gemaraju